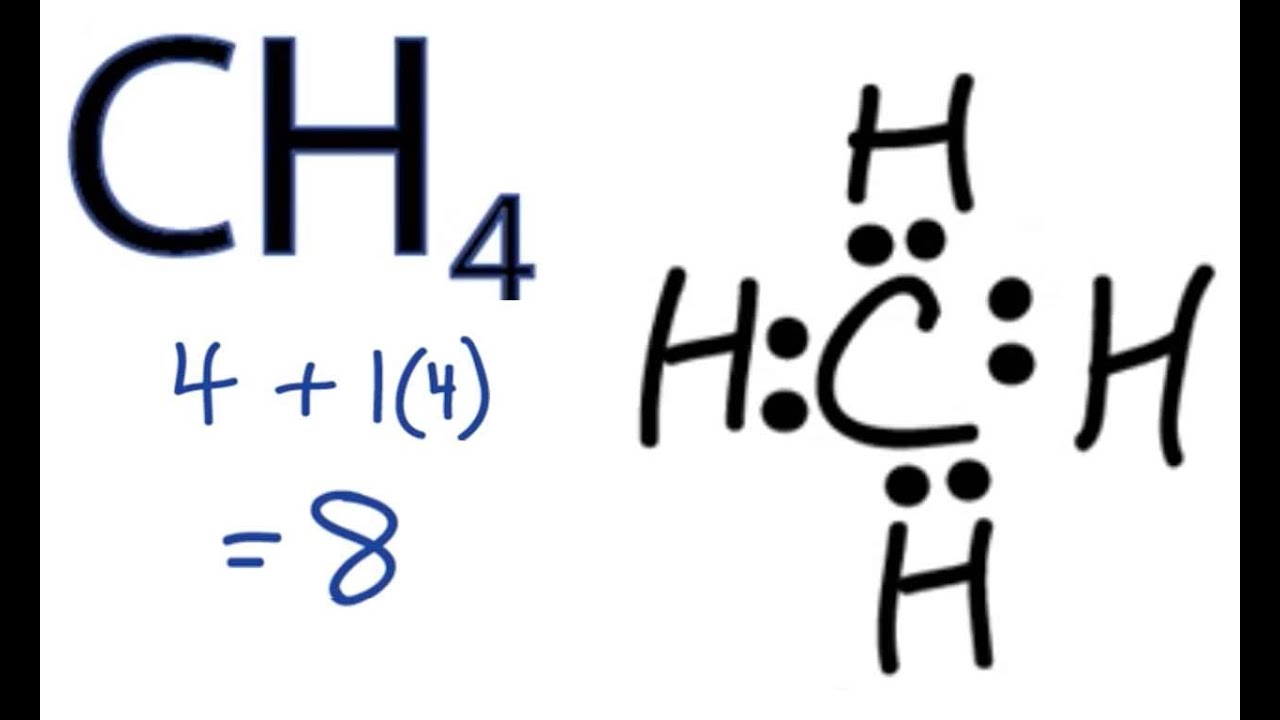The Secret Of Info About How To Draw Methane
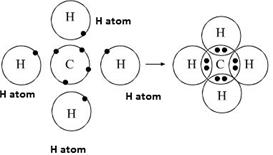
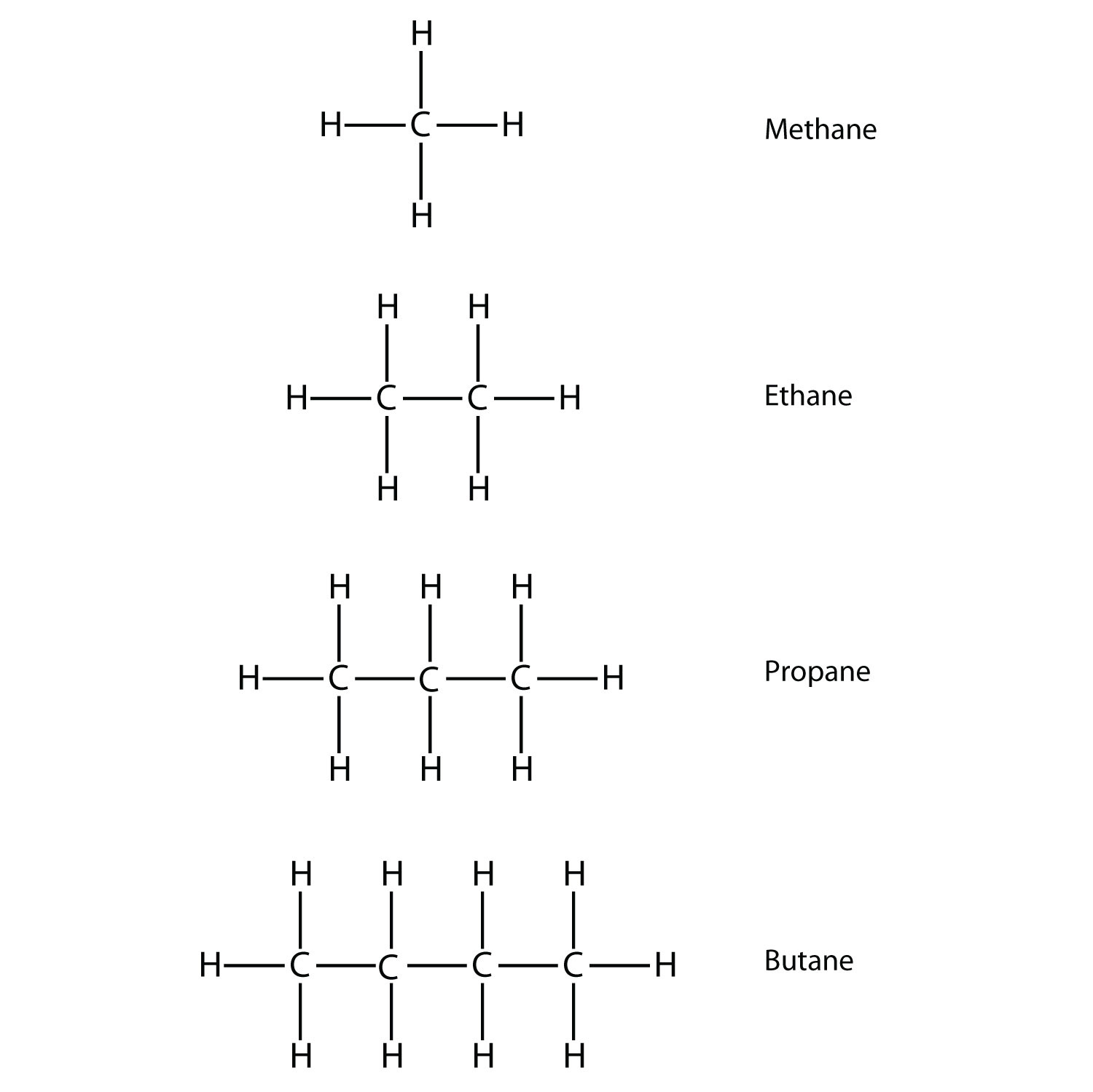
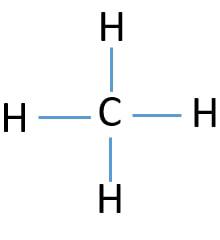
The total valence electron available for drawing the methane (ch4) lewis structure is 8.
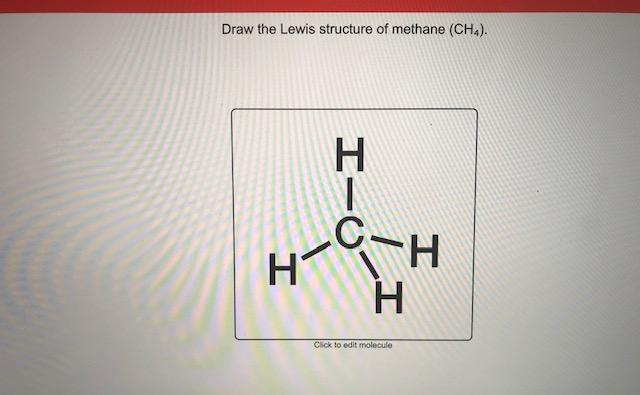
How to draw methane. Build a model of methane, ch4 draw methane using wedges to represent atoms in front of the page and dashed lines to represent atoms behind the page. How to draw the lewis dot structure for ch4: Ch4 is also called methane.
How many structural isomers can you draw for methane? For methane ch4 carbon should have 8 valence electrons each hydrogen should have 2 valenceelectrons 10. Draw methane (half of ethane's structure) duplicate the methane and draw bond between both.
How to draw methane,methanol,methanal,methanoic acidtags:formula of methanoic acid,difference between methanal andmethanol structure,methanoic acid,methanol. Rotate one methyl moiety by 180°. Name formula number of structural isomers;.
Draw an initial sketch of the molecule by placing the symbol of. For methane ch4 carbon should have 8 valence electrons each hydrogen should have 2 valenceelectrons 10. Open periodic table (ctrl+m), click the advanced tab, select alias from the bottom of the page (custom property button group), write c into the text field below then click the.
This video will go through the new method marvinsketch, chemaxon's chemical drawing tool uses for creating single step chemical reactions.learn more on our w. Then, we have eight hydrogen atoms, so we’re going to put a subscript of 8 after hydrogen. The steric number of the central atoms in methane is 4 which ensures that it has an sp 3 hybridization.
We will put c atom in the. Find the total valence electrons for the ch4 molecule. Make sure that the draw normal tool is enabled on the structure toolbar and that the carbon button is selected on the atoms toolbar click in an empty space to draw.